
A New Phosphate Fighter? Can Lanthanum change the way we attack excess phosphates in the aquarium? Daniel Knop investigates…
Letter From Europe
“This is an astonishingly effective substance, and my aim is to give the aquarium industry an incentive to develop appropriate products.”
Originally published in the November/December 2009 issue of CORAL Magazine
The regular partial water change is one of the key strategies in the control of slime algae. The usual recommendation is to change the water monthly or weekly, but Austrian Koralle writer Professor Ellen Thaler, who maintains a large number of reef fishes in her numerous aquariums for her ethological/animal behavior studies, swears by the daily change of a small percentage of the water volume. In a 130-gallon (500-L) tank, that would be all of 1½ gallons (5 L) of water, which can easily be topped up from a large container of previously prepared sea water. Proponents of the frequent water change contend that the benefits for reef aquariums become progressively greater the more frequently it is performed.
However, because the rise in phosphate can be controlled only to a limited extent by partial water changes, the use of an adsorber is generally required as well.
In this Web Bonus, I explore the subject of phosphate in the aquarium. In the future, I believe that the chemical element lanthanum may play an important role in phosphate extraction, especially for very large marine aquariums. This is an astonishingly effective substance, and my aim is to give the aquarium industry an incentive to develop appropriate products.
-Daniel Knop
Editor, 2017: To see one product that entered the aquarium marketplace after this publication by Daniel Knop, see Marc Levenson’s video discussing (and endorsing) a lanthanum chloride product he uses in his own reef aquaria.

The stony corals in the 20,000-gallon (76,000-L) reef aquarium of Joe Yaiullo grow vigorously. This view from above shows that the fishes are well-nourished and stony corals are thriving.
Foiling Phosphate: A Look at Lanthanum Chloride Use in the Aquarium
By Daniel Knop
First published as an online bonus to the the November/December 2009 issue of CORAL Magazine
In the last two decades, phosphate has come to be recognized as one of the central challenges of the reef aquarium hobby. I can still remember the 1980s very well, when the average coral reef aquarist didn’t measure either phosphate or magnesium concentrations – and in fact couldn’t measure them at all, as the appropriate tests were not yet available. Soft corals were popular; Litophytum arboreum and Nephthea species vied for popularity, and toilet-lid-sized Sarcophyton leather corals or huge Tridacna gigas were the pride of the aquarium owner (the giant clams were in fact T. squamosa, but hardly anyone knew this, as there was no literature available on giant clams back then). Then came the first HQI lamps, a regular quantum leap in aquarium lighting. And phosphate was anything but a problem. Happy days…
As the majority of coral-reef aquarists are aware, all this has now changed significantly. Soft corals are often no more than a peripheral interest in the marine aquarium hobby (which I think is a real shame), and coping with phosphate has become a serious concern that hangs over the hobby like a threatening rain cloud and takes a lot of the pleasure out of the brightly-colored stony corals that are now almost ubiquitous. The reason for this is that this nutrient has a negative effect on the physiology of zooxanthellate Scleractinia in two ways: on one hand, it encourages the proliferation of brown symbiotic algae in the coral tissue, masking the natural color pigments of the corals and causing the latter to turn brown. On the other hand, it restricts the calcium carbonate uptake necessary to enable the skeleton to grow.
It is true that nowadays effective control of the phosphate content is by and large feasible, as numerous firms offer phosphate-binding adsorbers. But in the case of larger reef tanks, the cost of the necessary water treatment can easily exceed affordable bounds, especially for aquarists on a tight budget. Those limited in how much they can spend on phosphate control now have two options: we can forgo delicate stony corals and limit ourselves to soft corals, which are less sensitive to raised phosphate levels – which, in the final analysis, is something that can be lived with. Or the aquarist can try feeding his or her fishes less often and less generously, thereby introducing less phosphate. In my opinion, this is not acceptable; at this point, the words of behavioral researcher Professor Ellen Thaler ring in my spiritual ear: if people are going to keep fishes in a glass box, then their diet should not be tailored to the available methods of water purification, but, on the contrary, the methods of water purification must be tailored to the nutritional requirements of the fishes. And she has a point: the food requirement of fishes is a constant, and aquarium technology should be the variable factor.
This was one of the reasons that I decided to look for a very economical method of lowering the phosphate content in the aquarium. Another reason was the fact that, as CORAL readers will know, at least since the sea squirt issue of this magazine (Volume 6, Number 4), I am exceptionally keen on filter-feeders. But the feeding of sea squirts, sponges, and tubeworms pollutes the aquarium water so heavily that the majority of aquarists don’t keep them intentionally and/or shy away from feeding a lot of suspended food and thus creating an environment in which such creatures can develop. But the reverse also applies: the dependence of filter-feeders on water-borne particulate food is so great that they can quickly die if they are inadequately fed. In fishes, hunger leads to increased aggression; in filter-feeders, it dramatically limits reproduction and the variety of species in the aquarium. To put it plainly: abundant feeding can encourage natural behavior in coral fishes, and in filter-feeders an increase in the number of species. Improved phosphate control is the key to achieving healthy stony coral growth.

Only when all the fishes congregate at feeding time does it becomes clear how enormous is the level of biological pollution affecting the 21,000-gallon (80,000-L) reef tank of Joe Yaiullo. Nevertheless, the phosphate content is so low that stony coral growth remains optimal.
During my intensive experimentation with sea squirts, I found out first-hand how drastically the concentrated feeding required increases the phosphate pollution of the water and how rapidly this restricts the development of organisms that metabolize calcium carbonate. I remain convinced that, even with more effective phosphate control, a basic distinction needs to be drawn between a stony-coral aquarium and a filter-feeder tank, and that combining these two very different groups of organisms in a single tank is not ideal. But I still wanted to try and find a particularly economical method of phosphate removal for the large reef aquarium.
What is lanthanum?
Granted, that was an unusually long introduction for an article of this kind. But sometimes it’s necessary. Now, however, for the important question: What is lanthanum?
Lanthanum, which has the chemical symbol La, was discovered in 1839 by Carl Gustav Mosander and is one of the “rare earths metals,” is probably of relatively little interest to the majority of aquarists. They may find it interesting to learn that lanthanum is used in human medicine to bind phosphate. In patients with kidney malfunction, whose blood has to be purified via dialysis (“blood-washing”), lanthanum is used to treat any excess of phosphate in the blood (hyperphosphataemia): lanthanum carbonate (under the proprietary name “Fosrenol”) is introduced into the stomach in the form of tablets, in order to bind phosphate from food before the body can take it up into the blood via the intestine. Were the phosphate to enter the blood, then it would have to be removed via sophisticated haemodialysis.
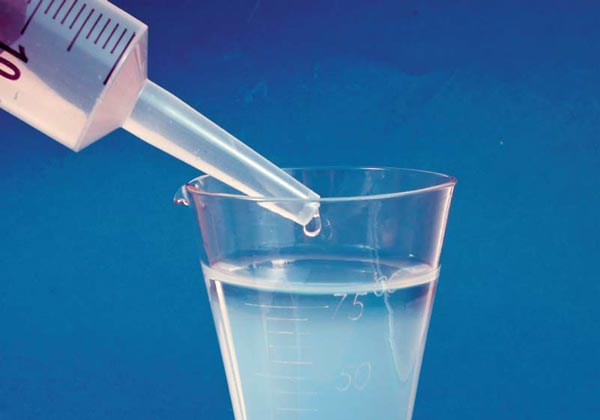
When lanthanum chloride is added drop-by-drop to phosphate-polluted aquarium water, it causes a whitish clouding in a matter of seconds, due to the flocculation of accumulated phosphate.
The phosphate-binding capacity of lanthanum matches that of aluminum, but without the toxicity that arises from long-term use. For this reason, lanthanum (in the form of lanthanum chloride) has for some time been used by the U.S. swimming-pool industry in order to prevent a build-up of phosphate in the water. A swimming pool, fitted with a sand filter, receives a daily, volume-dependent amount of the liquid preparation, which on contact with phosphate-laden water immediately forms tiny flocs (flakes of lanthanum phosphate – LaPO4 – precipitate), which are then trapped in the sand filter. Wouldn’t it be nice if we could do something of the sort in the coral-reef aquarium as well?
The good news is, we can. The bad news is that aquarium-hobby manufacturers have yet to develop a suitable filter for the purpose. In principle, the processes involved in binding phosphate via lanthanum are similar to the use of lime water: the dissolved phosphate precipitates out as tiny particles, which are then exported via the protein skimmer. As with calcium hydroxide, the effectiveness of lanthanum is greater the higher the phosphate concentration in the water, which means that lanthanum is particularly suitable for reducing a very high phosphate concentration, and less useful for lowering a slightly raised concentration to an ideal level or keeping it low via long-term application. That appears to remain the province of established phosphate adsorbers.
Proprietary phosphate-adsorbers
In consequence, it is to be expected that the phosphate adsorbers currently available on the market–for example, those based on iron oxide–will still have a meaningful role to play. For instance, after the slow (!) reduction of an extremely high phosphate concentration of, say, 1.5 mg/L to a value of 0.2 mg/L using lanthanum, conventional adsorbers could be used to lower the phosphate level further to the ideal range and keep it there, for example at 0.06 mg/L, the figure cited by Spotte (1979) for natural sea water. But I reckon that, because of its extremely low cost, lanthanum will usher in a new era in phosphate control, especially for larger and even very large reef aquaria, and may also be of interest for public aquaria. Even in all-fish tanks with a heavy metabolic turnover, it will be possible to control high phosphate concentrations and the resulting plagues of algae, given that the nitrate level can be easily regulated via a deep sand bed (DSB) filter.
Joe Yaiullo, Director of the Atlantis Marine World (Interview, CORAL September/October), has been using lanthanum chloride to regulate the phosphate content in his 20,000-gallon (76,000-L) reef tank for around three years, and throughout that period I have kept in touch with him on the subject, and have also visited his aquarium several times in person. He uses a liquid preparation called “SeaKlear Phosphate Remover,” which is diluted with water and added to the aquarium, and the LaPO4 is then taken out of circulation via the sand filter that forms part of his system. He does, however, use an iron-oxide-based phosphate adsorber in parallel. His results with lanthanum are exceptional, and although he still regards its use as experimental, he is convinced that the fantastic stony-coral growth in his very large coral-reef tank would not be possible without phosphate control using lanthanum, as the alternative, binding the phosphate on an adequately massive scale via conventional phosphate adsorbers, would quite simply be too expensive. And when this man says “fantastic stony-coral growth,” he knows what he is talking about, as he is forever dissolving the skeletal material of stony coral cuttings, taken during routine pruning, in the lime reactor, as there are too many to accommodate in other aquaria.
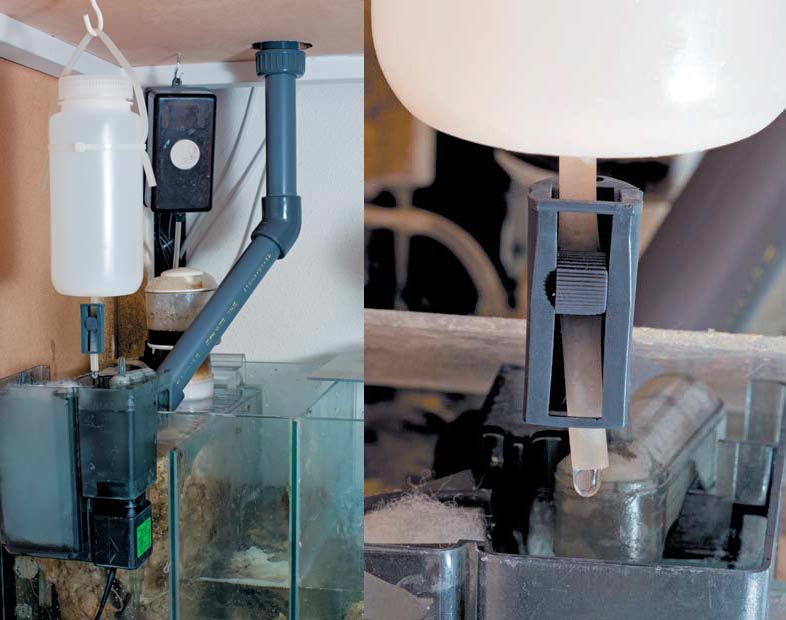
Experimental addition of diluted lanthanum chloride solution to an external filter filled with filter floss, feeding into a chamber of a filter tank containing a protein skimmer; within seconds of the beginning of the addition of lanthanum, the skimmer overflowed. The drip rate was regulated using a dosing clamp, such as is used for medical infusion, on the feeder tube.
Addition and removal of lanthanum
In my experiments, I introduced the lanthanum chloride solution with the aid of a dropper-bottle arrangement, at the rate of some two drops per second. But it would be equally possible to administer it with a proprietary dosing pump. As already mentioned, on contact with the phosphate in the water the lanthanum precipitates out rapidly as lanthanum phosphate and causes a whitish clouding, as I have shown experimentally in a glass container. If the water is left undisturbed, the particles gradually settle, but if the water is agitated they remain in suspension.

The filtrate was rinsed out of the floss into a glass; within a few minutes, a significant layer of sediment had accumulated at the bottom.
This means that the application needs to take place in a filter tank and that the resulting lanthanum phosphate must be removed immediately before the water reaches the aquarium itself and settles out there. The lanthanum phosphate will not dissolve in the aquarium water at the pH values typical of marine aquaria, but remain stable, so in order to prevent it from forming deposits it needs to be removed from the aquarium water. Only after the removal of the lanthanum phosphate flocs is the process of phosphate reduction complete.
One very simple option would be the application of lanthanum chloride directly to the aquarium (which I don’t advise!) and then filtering out the precipitate with a sand or diatomaceous filter, accompanied by very strong circulation of the water to prevent the flocs from settling out. In my view it is a far better idea to apply the lanthanum in a sump or filter chamber and then remove the product of the reaction via a protein skimmer plus subsequent fine filtration before returning the water to the aquarium.
I see two main ways of using liquid lanthanum chloride. One is similar to that used in the U.S. swimming-bath industry and also by Joe Yaiullo in the filter tank of his coral-reef aquarium: specifically, the chemical is added in much diluted liquid form, with subsequent filtering out of the precipitate. A sand filter is ideal for this, although Joe reports that where lanthanum is used, more frequent back-flushing is necessary.
I have experimented with filter floss and found that, using the proprietary fine nylon floss, by far the majority of the microflocs are removed from the water. The experimental use of cotton wool, which is considerably finer still, improved this result further, so that the remaining clouding in the aquarium was even less. However, the cotton wool had to be replaced within a day or two as the filter became clogged.

After six days of the addition of lanthanum, the filter was already exhibiting heavy deposits. The filter floss had been in use for only two days and was already extremely clogged with deposits, so that water could no longer pass through it.
What should perhaps be established experimentally is, for example, an answer to the question of which of the established methods of protein-skimming available on the market (reverse-flow with wood diffuser[s], pressurized with venturi, pinwheel, and so forth) is most effective at removing the LaPO4 flocs from the water. It might be possible to add the lanthanum in advance of the skimming, as Dr. Craig Bingman suggested in the 1990s for the addition of lime water, such that subsequent fine filtration wouldn’t be necessary at all.
Dosage
My experiments were intended to give me some idea of the phosphate-binding efficiency of lanthanum, the difficulties of removing lanthanum phosphate from the water, and also the effect of a short-term overdose. Joe Yaiullo always uses a single dose of 300 ml of lanthanum chloride for his 20,000-gallon (76,000-L) set-up, which he mixes with 5.25 gallons (20 L) of reverse-osmosis water before drip-feeding this solution into a sump. From there, the solution passes through the protein skimmer before reaching the aquarium. The protein skimmer is generally able to remove a significant amount of the lanthanum phosphate, but Joe believes the sand filter has a far higher capacity for removal of this compound.
Joe’s lanthanum chloride dosage works out at 0.00375 ml/L . In a 132-gallon (500-L) aquarium that would equate to a dose of 1.875 ml, and 3.75 ml in a 264-gallon (1,000-L) tank. I, on the other hand, used 3.5 ml for a tank volume of 132 gallons (500 L) – that is, almost twice Joe’s rate of 0.00375 ml/L. Unlike Joe, I was able to detect significant damage to the few specimens of stony corals in the tank, as corals of the genera Acropora, Montipora, and Porites died. This was, however, a filter-feeder tank with intensive feeding and a dramatically elevated phosphate concentration, so that the few stony corals present were undoubtedly not in optimal condition. They were adapted to a phosphate concentration of 1.6 mg/L, and in consequence grew slowly, and it seems that when this concentration dropped to 0.3 mg/L within a few days as a result of the drastic treatment, the complex physiological processes that take place in these corals were thrown out of balance. For this reason I cannot advise too strongly that treatment with lanthanum should proceed very slowly in an established stony-coral aquarium, with the objective being to lower the phosphate concentration over the course of weeks or months. In addition, this should be achieved by reducing the dose rather than the frequency of treatment; in other words, by using a minimal dose on a daily basis rather than a high dose once per week. In some cases, the required dosage may consist of only a few drops of the lanthanum solution, and this should then be greatly diluted with water before use. One should not underestimate the enormous phosphate-binding capacity of lanthanum; a one-off high dose can have a dramatic effect on the phosphate content of the water and also on the physiological processes of the corals.
Phosphate-binding capacity
In short, the phosphate-binding effect is truly breathtaking. As I already mentioned, the phosphate content of my experimental tank, in which my sea squirt work took place, was 1.6 mg/L (Merck phosphate test). After four single daily doses of lanthanum chloride (3.5 ml apiece, diluted with 500 ml water and drip-fed into an open-topped external filter hanging on the filter tank), I decided to see if there was any measurable success yet. The reading was already down to 0.3 mg/L! However, I should be add that this related only to dissolved phosphate, while as a rule by far the majority is present in the form of deposits of undissolved calcium phosphate, which passes into solution in the water after the concentration has been lowered. In consequence, I now stopped the addition of lanthanum in order to record the subsequent rise. And, lo and behold, after another four days the reading was up to 1.5 mg/L–not all that fast.
Using lanthanum in liquid form
It will be possible to use lanthanum chloride in liquid form for phosphate control in the coral reef aquarium as soon as effective methods of removing the LaPO4 flocs from the water are developed. Using suitable filter equipment, it appears to be perfectly feasible to prevent the occurrence of deposits in the aquarium; hence – as far as I know at present – the method can be used without risk, as long as the phosphate concentration is not excessively high and the treatment takes place at a sufficiently slow rate. However, the permanent lowering of the concentration requires long-term treatment with regular applications, and, as mentioned earlier, it must not be forgotten that the effectiveness of this method diminishes once the phosphate concentration in the aquarium water has been significantly reduced, for which reason conventional phosphate binders should be used in addition, in order to achieve the ideal level and maintain it in the long term.
Using lanthanum via filter matting
Another method that I can envisage in principle–although to the best of my knowledge this mode of application does not yet exist–would be the permanent presence of lanthanum on the fibers of filter matting, with the aid of a carrier substance.
I envisage such a lanthanum filter matting as being similar to “Poly-Filter,” synthetic matrix pad made by Poly-Bio-Marine in Reading, Pennsylvania, whose fibers are coated with a proprietary chemical material that reacts with substances in the water and absorbs or binds them. In this way, it might be possible to bind phosphate using lanthanum, without the resulting LaPO4 being able to move freely in the water.
Such a lanthanum filter matting could be placed in a specially constructed filter in which, after contact with the lanthanum, the water passed through several more layers of dense filter material to trap any LaPO4 that might escape. As well as liquid lanthanum chloride preparations such as “SeaKlear,” this chemical is also available in powder form, which might facilitate the development of such a product.

Poly Filter (above), a medium from the USA, whose fibers are coated with adsorptive material (see macro-photo, below) that can bind a wide variety of dissolved substances – could this principle be used for using lanthanum in the marine aquarium?
Lanthanum granulates?
Consideration should also be given as to whether ions other than chloride – carbonate, for example – are suitable partners for lanthanum for use in the marine aquarium. Lanthanum carbonate is non-toxic and has an enormous phosphate-binding capacity. Perhaps it will be possible to use lanthanum carbonate to create a solid filter granulate that won’t lead to flocculation but instead adsorb phosphate, and can then simply be removed from the water. It may even be that a thin coating on an existing granulate base – calcium carbonate, for example – might do the trick. And this type of coating, with a different carrier medium, applied to standard calcium carbonate granulate, might perhaps provide a solution – this is an area where the product developers in the marine aquarium hobby industry can experiment. Above all, it can answer the question of whether lanthanum carbonate in solid form can adsorb phosphate effectively, or whether it needs to be dissolved by an acid, as with the Fosrenol medication in the stomach of the human body.
Ideas for the development of lanthanum filtration for marine aquaria
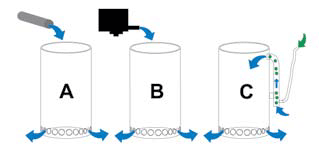
Feeding water to the suggested lanthanum filter: A via an existing supply pipe (e.g., branch from an outflow pipe, branch from return pipe, inter alia); B via a very low-turnover powerhead; C via an airlift.
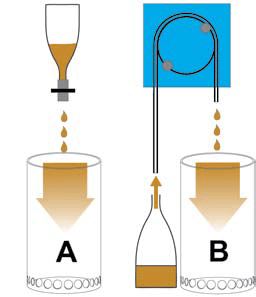
The addition of lanthanum to the proposed lanthanum filter: A dropper bottle with airline tubing and clamp; B dosing pump with clamped tube.
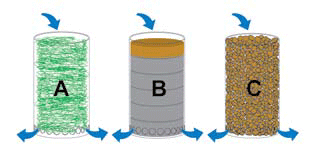
Filtration methods: A fine nylon filter floss for use with a liquid lanthanum preparation; B the suggested filter pads with lanthanum coating on the fibers (see text), followed by fine filter pads; C the suggested granulate with lanthanum coating (for example lanthanum carbonate).
The first diagram shows possible methods of feeding water to the lanthanum filter, the second illustrates two options for the addition of lanthanum, and the third explains three different potential filtration methods.
The through-put of the filter should be very weak, as this will increase the retention capacity of the filter media. In my opinion, a filter situated at the inlet to a powerful pump will not be effective for filtering out the finest micro-particles of LaPO4 flocculate, as the powerful suction will pull a lot of the filtrate straight through the filter – in this case, less is more. Feeding the filter with water via the outflow pipe of a protein skimmer also makes no sense, as in my view the protein skimmer should be fitted after the lanthanum filter, in order to remove the maximum possible percentage of the resulting micro-particles of flocculate from the water.
Possible risks
The liquid phosphate binder “SeaKlear” is available cheaply by the gallon from swimming pool suppliers, but I would advise the hobbyist against experimentation; the risks involved in using lanthanum chloride in the marine aquarium are not yet known with absolute certainty, at least as regards long-term usage and the effects on very delicate organisms.
A further minus point is the tendency of lanthanum chloride to form deposits on glass or plastic surfaces.

After six days of the addition of lanthanum, there were already noticeable deposits in the pump housing. Briefly dipping the right-hand part of the housing in 37 % hydrochloric acid quickly removed the deposits. For safety reasons, a mild acid such as vinegar can be used instead.
This very hard, whitish layer can only be removed with difficulty mechanically, and hence has to be cleared away by chemical means. This is feasible, albeit inconvenient, in the impeller housings of current pumps, but can cause problems on the aquarium panes, particularly in the case of acrylic. (In my experience, a permanent slight coating of algae on the pane can largely prevent these deposits, while my experiments have shown that when the addition of lanthanum is considerably increased, a sort of “gray veil” rapidly develops on panes constantly kept clean, and this could be removed only with a very good blade-type algae scraper.) This is another reason why the addition and removal of lanthanum should take place in a separate filter tank. In practice, the deposits on the aquarium panes may turn out to be significantly less, as a considered lanthanum dosing regime would undoubtedly differ from my experimental high dose, but I suspect that the danger of deposits on the aquarium glasses will lead some aquarists to instead stick to proprietary phosphate adsorbers – those based on iron oxide, for example.
But in my view, these, and perhaps many more as-yet-unknown disadvantages, will be more than compensated by the really fabulous phosphate-binding capacity of lanthanum. Moreover, in the roughly three years during which Joe Yaiullo has been using this method as the chief means of removing phosphate in the Atlantis Marine World reef, no problems have occurred that might be attributed to the use of lanthanum chloride – despite the acrylic viewing panes.
Nevertheless, I must once again expressly advise hobbyists against conducting their own experiments with lanthanum chloride, as well as devising the necessary equipment for its addition and removal. Instead, I suggest they wait until the aquarium-hobby industry has developed appropriate methods. Word travels fast, and I would not want to be the cause of countless coral-reef aquarists experimentally pouring chemicals into their living-room reef aquarium to see what happens. This article, with its suggestions regarding the use of lanthanum in the reef aquarium hobby, may make members of the aquarium industry aware of the possibilities; and because phosphate is a considerable problem in the marine aquarium hobby, it shouldn’t be long before innovative companies are offering suitable proprietary products and appropriate filter equipment that will actually remove by far the majority of the lanthanum phosphate microflocs from the aquarium water. I believe that the marine aquarium-hobby industry will find much scope for innovation in the field of lanthanum, perhaps leading to numerous new and useful products. In this way, phosphate control could become easier and cheaper, so that its implementation might become the norm.
This would perhaps enable many stony-coral fans to shrug off their current timidity about feeding their fishes, and in addition it might lead to more people experimenting with maintaining azooxanthellate corals and other filter-feeding invertebrates, regardless of the abundant floating food that they require (Knop 2009). And that could represent yet another quantum leap in the reef aquarium hobby.
New Phosphate Fighter Downloadable PDF
References
Knop, D. 2009. Sea Squirts in the Aquarium. CORAL Volume 6, Number 4, July/August 2009.
Spotte, S. 1979. Seawater aquarium, the captive environment. John Wiley & Sons, New York.
More on Lanthanum Chloride
Levenson, Marc. 2017. Video: Melev’s Reef Explains Phosphate Rx (A Lanthanum Chloride Product)








Very interesting. I am using lanthanum chloride to aid the removal of Po4 from acid-etched dry rocks. A couple of thoughts as to its use in hobby aquariums.
Would it be possible to
a) Drip the low concentrate lanthanum into the skimmer chamber and the skimmer skim it out?
b) Have a two-stage reactor with a drip feed of lanthanum in the reaction part and the water flowing though a sand filter in the second part.
c) Some form of centrifugal chamber to spin out the flocks.
I think the use of Lanthanum Chloride is very useful in binding Phosphates for removal but one thing must be taken in consideration. Zebramosa Tangs for some reason react negatively to Lanthanum. They will show signs of stress with rapid respiration which can result in death if used with too high of a doseage