When You Wish Upon a Staur…ozoan
What is that stalked animal that has invaded my reef?
By Ronald L. Shimek, Ph.D.
Excerpt from CORAL Magazine, Volume 16, Number 6 (N/D 20918).
Catch a Falling Staur…
I have been fortunate in the long and meandering safari that is my life to have spent some time examining and investigating the often overlooked, the small, the unknown, the mysterious invertebrate animals, getting lost along the way in their beauty. Many of them are astoundingly beautiful. They delight the eyes and are often interesting beyond all expectation. They stimulate the mind and refresh the soul. So, “Wetward Ho!” to a small group of interesting, attractive, largely unknown, and somewhat bizarre Cnidarians which most aquarists have never seen, read, or heard about, but which might show up in their tanks from odd collections or habitats. When I started to learn about these Cnidarians, these animals were known to zoologists as the Order Stauromedusa (stalked jellyfish) in the Class Scyphozoa (true jellyfish) of the Phylum Cnidaria (corals, sea anemones, jellyfish).
Other than a basic structure, few things about them were conclusively known, and those were known only for a couple of species. It was not clear which properties were general to the group and which were unique to the species being examined. They have all the properties necessary to conclude they were Scyphozoans; nevertheless, they lack all swimming stages–notably the swimming medusa stage, the fact of which must somewhat embarrassing for a medusazoan animal. Their planula larvae are bizarre: they are not like small flatworms. They don’t glide around but have eight linear internal or endoderm cells, surrounded by a layer of smaller ectoderm cells. The planula moves around, rather like a small earthworm, expanding and contracting the various cells to provide a sort of peristaltic locomotion. In at least some staurozoans, the planula can asexually produce other planulae, presumably by fission of some sort. Several researchers have noticed that planulae may aggregate and secrete a protective material over themselves, possibly to form an overwintering stage.
Once the larva picks a spot to settle, it metamorphoses and grows from the planula into a polyp, called the stauropolyp, which looks like a small hydrozoan, having only four tentacles. The stauropolyp grows and metamorphoses directly into the adult without asexual reproduction. The stauropolyp grows to form a simple polyp with only four primary tentacles. As it continues to grow, the upper part of the animal metamorphoses into a small attached medusan, called a stauromedusan. For a time, the animal is actually part-polyp and part-medusa, neither part of which is typical polyp or medusa. Later the stauropolyp undergoes its own internal metamorphosis, forming the four internal cavities characteristic of the adult animal.
These animals are distinctive enough to have been elevated in taxonomic rank to Class Stauromedusa, the stalked jellyfishes, one of six recognized Classes in the Phylum Cnidaria, animals that typically have cnidocytes or stinging cells used in prey capture.

Excerpt from CORAL Magazine, Volume 16, Number 6 (N/D 20918).
Aquarium Medusae
Aquarists are now getting experience in keeping swimming medusae, as a small variety of medusae have been found to survive well in specialized tanks. These desired medusae, which include Aurelia labiata, the Moon Jellyfish, are generally Scyphozoans. Surprisingly, one species of the almost wholly dangerous Cubozoans, the Caribbean Mangrove Box Jelly, Tripedalia cystophora, is harmless and is also occasionally offered for sale. The generally unintended medusae found in aquariums or food-culture vessels are the common hitchhiking hydroids or Hydrozoans, including the wall-walkers, Staurocladia oahuensis, and the bent-tentacled Cladonema californicum.
Nevertheless, while all of these species are very good medusazoans, they are all representatives of more recent lineages than that of the Staurozoa. So the question, I suppose, is what do Staurozoans look like? Fortunately, they are generally easily recognizable, with one caveat: “Ease of recognition, like beauty, is in the eye of the beholder.”
Sometime after spotting an animal in your reef or on a piece of live rock, or even intentionally bringing one home, there comes the first attempt at identification. The identification process given here is sort of loosey-goosey, but it works.
The intent is not to identify the critter to species, but just to give a reasonable, though tentative verification that the organism is, or is not, a Staurozoan. This is the first step in the long process of actual identification. Hopefully this is occurring without any pressure; however, for any truly unknown organism, there is always the justifiable worry about the occasional specimen being dangerous, either to the aquarist or the system’s established organisms.
The first question to answer, “Is it a polyp?” is tricky; stauromedusan individuals are not actually polyps, but they do look somewhat like them. The question, then, is, “Does it appear to be a polyp or something like a polyp?” If the answer is no, turn off the lights and go have a libation of your choice, because the animal, or whatever it is, probably isn’t a stauromedusan, or the organism is badly damaged, or the definition of polyp that was used needs to be checked. If yes, then closer examination is necessary.
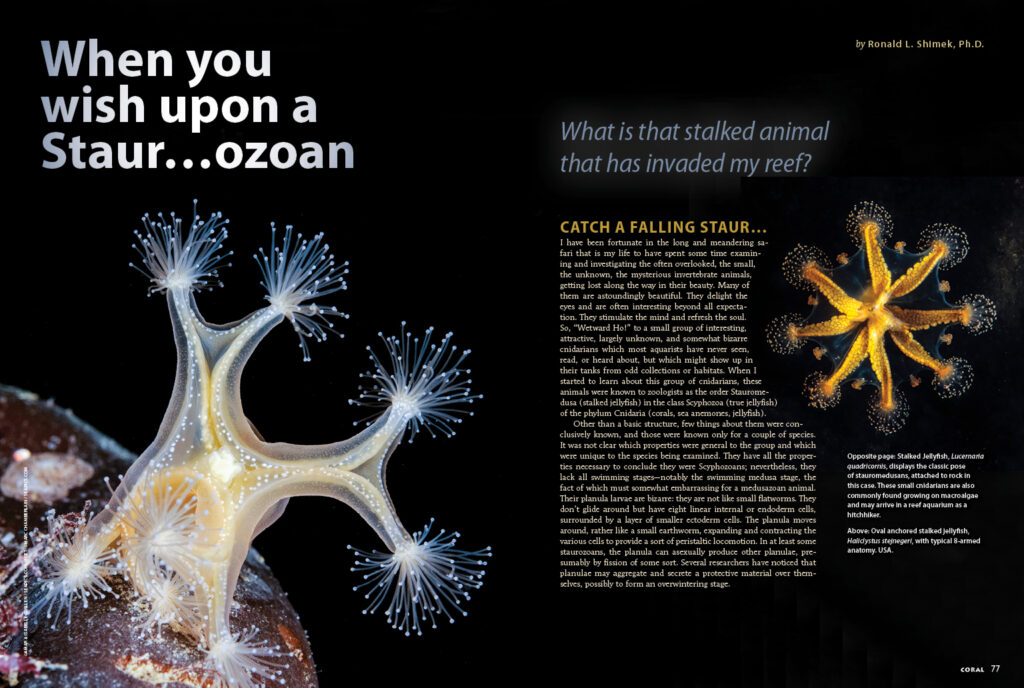
Stauromedusan “polyps” differ from “normal” Cnidarian polyps in a number of ways, which shouldn’t come as a shock, given they are not polyps, but medusae. As in most medusae, they are tetramerous, having four divisions or multiples of four. The basic shape, if seen looking down on a vertically-extended animal, is that of a trumpet- or funnel-shaped animal, which the observer is examining by looking into the bell of the trumpet which has been divided into quadrants or octants by long extensions, each often ending in a “puff” ball of small extended tentacles. The funnel’s conical apex is under the funnel’s cone or bell and is connected to the substrate by a thickened tube leading from its bottom. There is a terminal pedal or adhesive disk holding the animal in place. Of the approximately 50 known species, most reach adult sizes of 1 to 4 cm (approximately 0.5 to 1.5 inches).
Below the center of the trumpet’s bell, in what would be the apex of a swimming medusa, a thickened, hollow tube connects the bottom of the lateral or upward-facing bell to the substrate. This tube, continuing upward through the bell’s center, terminates in a rounded surface with a slit or round opening, the mouth, in the center. At the bottom of the bell is the point of origin of four or eight large, thickened radial extensions, corresponding to the radial canals found in a regular downside-down medusae. These arise from the sides of the central tube and extend upward in the bell. In a manner somewhat like the situation in Cubozoans, the upper end of each of these is the primary site of tentacle attachment. Unlike in Cubozoans, where the tentacles hang down, the tentacles originating from the stauromedusans’ corners project out in all directions.
If such structures are not seen, it is unlikely the organism is a stauromedusan. If they are seen, the organism is likely a stauromedusan, and comparison to the images in this article should be sufficient to verify that fact. Further identification might be done comparing the organism to the images or references, now that its identity is basically known, and other anatomical structures may be seen and identified. Compared to the other Cnidarian groups, there are not many staurozoans; only about fifty species have been described. Consequently, if it is a stauromedusan, the odds are pretty good that it will look somewhat like one of the organisms pictured.
Natural History & Discovery
Authors of references and text books, if they discuss staurozoans at all, write that they are generally found in shallow-boreal or temperate seas, although a couple of species have been recently described from the coral-reef tropics and deep seas. I think if someone looks at those same distribution data with a bit of a “history of science” in their approach, it will be evident that staurozoans are distributed either near well-established marine laboratories or in areas where a lot of marine biological work has been done over the last century or so, often in areas where expeditions with mandates to find animals have been mounted.
Similarly, the few staurozoans found in the tropics have typically been found near where marine stations have recently been established, typically within the last 30 years or so. They are also being found where people are now regularly spending time sampling to document the effects of environmental degradation, or dredging in very deep water or using submersibles to collect small animals. In other words, it appears that their known distributions are better understood as maps of scientific search effort than they are of the actual staurozoan distributions. The average beach-walking amateur naturalist is not looking for these rather odd critters and will therefore not likely find them.
Consequently, I think many staurozoan species remain to be discovered, probably by divers in waters from 3 to 18 m (10 to 60 ft) in depth, most likely in the tropics. Staurozoans are generally attached to rocks, macroalgae, or other animals, in surge zones or in areas with variable and strong water currents. They are adapted to catch plankton or animals floating in, or dislodged by, the changing currents. In many regards they feed rather like normal medusae, except that they don’t drift around to catch prey; they catch drifting prey that comes to them.

Excerpt from CORAL Magazine, Volume 16, Number 6 (N/D 20918).
Captive Care
In an aquarium they would undoubtedly need relatively strong, variable currents, and would probably detach from the substrate and die if the water currents were continuously steady or too slow. In nature, they feed upon small crustaceans such as amphipods and copepods, but some species have been known to eat peppermint shrimp. The shrimp bumps into the bell and sticks to it, not by using nematocysts, but by using an adhesive substance. The stauromedusan then pivots and rolls over so that the shrimp is trapped under its bell. The bell is forced down on the shrimp, and eventually the shrimp is eaten. About four or five hours later, the shrimp’s carapace is expelled from the medusa’s mouth. In an aquarium, Artemia of various sizes, or frozen plankton that is thawed and gently added upstream of them in the water flow, should suffice. If the system contains peppermint shrimp or other similarly-sized ornamental shrimp, they may be in peril. All known species lack zooxanthellae, and lighting would be immaterial to them; however, many of them can be found, at least some of the time, attached to algae, which will need the appropriate lighting.
Probably all of them can move around; however, some individuals have been reported to not be able to reattach once they have detached. As always in such situations, the animals’ reattachment is likely to be determined more by the animals’ health and appropriate attachment situations (such as suitable attachment substrata and current flows), than their true “ability” to reattach.
Relatively small animals such as staurozoans are often inadvertently brought into the reef aquarium hobby by collectors who are searching for other things and collect these polyp-like animals in passing when specimen corals or other animals are collected. As long as the rock or animal they are on remains relatively undamaged, the stauromedusans should be able to survive the trip to the home aquarium. Particularly if the hitchhikers are small, or attached in rock cracks or crevices, the animals may stay alive and in good condition until a hobbyist purchases the specimen and drops it into a quarantine tank. If they are then identified, put into an appropriate current regime, fed well, and kept as a typical oddball critter in a good aquarium, they should survive without any problems. (According to some sources, they have short lifespans, living for about a year.) If, at some later time, the aquarist is lucky enough to have a staurozoan reproduce in their tank or refugium, a new species can be added to the pantheon of critters we can watch and enjoy.
References:
Collins, A. G. and M. Daly, “A New Deepwater Species of Stauromedusae, Lucernaria janetae (Cnidaria, Staurozoa, Lucernariidae), and a Preliminary Investigation of Stauromedusan Phylogeny Based on Nuclear and Mitochondrial rDNA Data,” The Biological Bulletin 208, no. 3 (June 2005): 221-230.
Han, J., Kubota, S., Li, G., Ou, Q., Wang, X., Yao, X., Shu, D., Li, Y., Uesugi, K., Hoshino, M., Sasaki, O., Kano, H., Sato, T. and Komiya, T. 2016. Divergent evolution of medusozoan symmetric patterns: Evidence from the microanatomy of Cambrian tetramerous cubozoans from South China. Gondwana Research 31:150–163. doi:10.1016/j.gr.2015.01.003.
Iten, H., Marques, A., Leme, J., Pacheco, M. and Simões, M. 2014. Origin and early diversification of the phylum Cnidaria Verrill: major developments in the analysis of the taxon’s Proterozoic–Cambrian history. Palaeontology 57:677–690. doi:10.1111/pala.12116.
Kahn, A.S., Matsumoto, G.I., Hirano, Y. M. and Collins, A.G., 2010. Haliclystus californiensis, a” new” species of stauromedusa (Cnidaria: Staurozoa) from the northeast Pacific, with a key to the species of Haliclystus. Zootaxa 2518: 49–59.
Katsuki, T. and Greenspan, R. 2013. Jellyfish nervous systems. Current Biology 23:R592–R594. doi:10.1016/j.cub.2013.03.057.
Kayal, E., Roure, B., Philippe, H., Collins, A. and Lavrov, D. 2012. Cnidarian phylogenetic relationships as revealed by mitogenomics. BMC Evolutionary Biology 13:1–18. doi:10.1186/1471-2148-13-5.
Larson, R.J., 1980. A new stauromedusa, Kishinouyea corbini (Scyphozoa, Stauromedusae) from the tropical western Atlantic. Bulletin of Marine Science, 30(1), pp.102-107.
Larson, R.J., 1988. Kyopoda lamberti gen. nov., sp. nov., an atypical stauromedusa (Scyphozoa, Cnidaria) from the eastern Pacific, representing a new family. Canadian Journal of Zoology, 66(10), pp. 2301-2303.
Liu, A., Matthews, J., Menon, L., McIlroy, D. and Brasier, M. 2014. Haootia quadriformis n. gen., n. sp., interpreted as a muscular cnidarian impression from the Late Ediacaran period (approx. 560 Ma). Proceedings of the Royal Society B: Biological Sciences 281:20141202. doi:10.1098/rspb.2014.1202.
Liu, A., Matthews, J., Menon, L., McIlroy, D. and Brasier, M. 2015. The arrangement of possible muscle fibres in the Ediacaran taxon Haootia quadriformis. Proceedings of the Royal Society of London B: Biological Sciences 282:20142949. doi:10.1098/rspb.2014.2949.
Miranda, L. S., and M. A. Carlos. 2016. Hidden impacts of the Samarco mining waste dam collapse to Brazilian marine fauna – an example from the staurozoans (Cnidaria). Biota Neotrop. [Internet]. 2016 [cited 2018 Apr 16] ; 16( 2 ): e20160169. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1676-06032016000200401&lng=en. Epub Apr 08, 2016. http://dx.doi.org/10.1590/1676-0611-BN-2016-0169.
Miranda, L., Collins, A. and Marques, A. 2013. Internal anatomy of Haliclystus antarcticus (Cnidaria, Staurozoa) with a discussion on histological features used in staurozoan taxonomy. Journal of Morphology 274:1365–1383. doi:10.1002/jmor.20185.
Miranda, L., Collins, A. and Marques, A. 2015. Is Haootia quadriformis related to extant Staurozoa (Cnidaria)? Evidence from the muscular system reconsidered. Proceedings of the Royal Society of London B: Biological Sciences 282:20142396. doi:10.1098/rspb.2014.2396.
Miranda, L. S., Morandini, A. C. and Marques, A. C., 2009. Taxonomic review of Haliclystus antarcticus Pfeffer, 1889 (Stauromedusae, Staurozoa, Cnidaria), with remarks on the genus Haliclystus Clark, 1863. Polar Biology, 32(10), pp. 1507-1519.
Miranda, L. S., Morandini, A. C. and Marques, A. C., 2012. Do Staurozoa bloom? A review of stauromedusan population biology. Hydrobiologia, 690(1), pp. 57-67.
Miranda, L. S., Haddad, M.A., Mills, C. E. and Marques, A. C. 2012. Lucernariopsis capensis Carlgren, 1938 (Cnidaria, Staurozoa) in Brazil: first record outside its type locality in South Africa. Zootaxa 3158:60-64.
Miranda, L. S., Collins, A.G., Hirano, Y. M., Mills, C. E. and Marques, A. C., 2016. Comparative internal anatomy of Staurozoa (Cnidaria), with functional and evolutionary inferences. PeerJ, 4, p.e2594.
Miranda, L. S., García-Rodríguez, J., Collins, A.G., Morandini, A. C. and Marques, A. C., 2017. Evolution of the claustrum in Cnidaria: comparative anatomy reveals that it is exclusive to some species of Staurozoa and absent in Cubozoa. Organisms Diversity & Evolution, 17(4), pp. 753-766.
Miranda, L. S., Mills, C. E., Hirano, Y. M., Collins, A.G. and Marques, A. C., 2017. A review of the global diversity and natural history of stalked jellyfishes (Cnidaria, Staurozoa). Marine Biodiversity, pp. 1-20.
Miranda, L. S., Branch, G. M., Collins, A.G., Hirano, Y. M., Marques, A. C. and Griffiths, C. L., 2017. Stalked jellyfishes (Cnidaria: Staurozoa) of South Africa, with the description of Calvadosia lewisi sp. nov. Zootaxa 4227 (3): 369–389
Miranda, L. S., Hirano, Y. M., Mills, C. E., Falconer, A., Fenwick, D., Marques, A. C. and Collins, A.G., 2016. Systematics of stalked jellyfishes (Cnidaria: Staurozoa). PeerJ, 4, p.e1951.
Park, T., Woo, J., Lee, D.-J., Lee, D.-C., Lee, S., Han, Z., Chough, S. and Choi, D. 2011. A stem-group cnidarian described from the mid-Cambrian of China and its significance for cnidarian evolution. Nature Communications 2:442. doi:10.1038/ncomms1457.
Zagal, C.J. 2004. Diet of stauromedusa Haliclystus auricula from southern Chile. J. Mar. Biol. Ass. U.K. 84:337-340, 10.1017/S0025315404009245h





Hi Ron,
Terrific article – fun & informative! I’ve never seen one of these although there are apparently several species in my area; I’ll have to pay more attention.
I’ve been trying to contact you recently but your wisp address doesn’t seem to work (neither did LinkedIn). Could you drop me a note with your new address?
Observantly yours,
Alex