
Biologist Eric Cassiano inspects an 800-gallon (3,028-L) Blue Tang (P. hepatus) broodstock tank at the University of Florida’s Tropical Aquaculture Laboratory. Broodstock tanks are supplied with a circular flow of water that brings pelagic eggs toward the collection bar (left foreground) and egg-collecting tank (lower left).
With detailed coverage of the world’s first successful breeding of the Pacific Blue Tang, Paracanthurus hepatus, being released in our September/October 2016 issue of CORAL Magazine, we thought it fitting to furnish our readers with a retrospective excerpt from CORAL. Just 3 years ago, in our July/August 2013 issue TANGS, we featured The Tang Challenge – Inside the global push to rear captive-bred surgeonfishes by Matthew L. Wittenrich, Ph.D., where he provided insight into the state of what is now historical research at the University of Florida’s Tropical Aquaculture Laboratory. We hope you enjoy this look back, and hope it provides a deeper appreciation for all that went into accomplishing what many considered impossible—breeding Dory. -Matt Pedersen
The Tang Challenge
Inside the global push to rear captive-bred surgeonfishes
Article & images by Matthew L. Wittenrich, Ph.D.
IN THE HEART OF THE PHILIPPINES: The shriek of the diesel engine pierced the evening sky as it strained and shook in its mount, trying to build up speed. The smoke billowing from the bowels of the old apparatus was thick in the bar, but drifted quickly across the Mayor’s bamboo table with the seaward breeze. It was hard to hear over the hum of the generator, but I thought he said, “No Blue Tang, no fish.”
A flickering incandescent bulb swung like a pendulum from a bare cord above the table, casting its yellow beam across the faces of a dozen or so Filipino fishers. The conversation was slow and interrupted often by the strings of a guitar and some very good English karaoke, but I was finally able to piece together a story about Blue Tang, known as the Palette, Pacific Yellowtail Blue Tang, and, most commonly in North America, the Hippo or Regal Tang (Paracanthurus hepatus), and how it influences the lives of these fishers on a tiny island in the Philippines.
“So popular, this fish…we can’t sell anything we catch unless we have Blue Tang,” the Mayor said. He explained that the Blue Tang is so sought-after by the Asian aquarium trade that these fishers couldn’t sell their other catches—neither hermit crabs nor clownfishes—to middlemen or exporters unless a quota for Blue Tang was included.
I have been thinking of this story a lot lately, as pressures seem to mount on those of us trying to find the secrets to completing the life cycle of tangs in captivity. There is no doubt that surgeonfishes are popular and hugely influential in the lives of those who collect, sell, buy, and try to rear them, but how close are we to captive-bred tangs that could potentially change the industry and the lives of those who depend on it.
UNSOLVED PUZZLE
Although it has been done with many popular groups of marine fishes, such the anemonefishes, gobies, and even angelfishes, very few hobbyists and research labs have undertaken the challenge of spawning and rearing tangs. Over the last decade or so, many rumors have surfaced claiming that someone was the first to rear a tang through the larval stages to metamorphosis and settlement. The aquatic community would come alive with chatter and excitement, only to be let down some time later when proof of a rumored accomplishment failed to surface.
Stories like this continue today. Rearing a tang in captivity will surely be a thrilling accomplishment for the marine aquarium community, but it hasn’t happened yet. Some organizations are close, and the puzzle is quickly unraveling for a few species, like the Blue Tang. Deciphering and conquering a pelagic larval stage estimated at 50 to 90 days and dealing with some of the tiniest, most delicate, and most selective feeders thus far encountered in marine fish breeding has culturists scrambling and starting over at the beginning, as the methods developed for other difficult-to-rear species have proven largely ineffective.
At the University of Florida’s Tropical Aquaculture Laboratory (TAL) we have recently focused our efforts on understanding tang larvae. Rising Tide Conservation (see CORAL, March/April 2012) partners such as TAL were initially funded to collect eggs spawned in large public displays, transport them to the lab in Ruskin, on Tampa Bay on Florida’s west coast, and document larval rearing of prospective species.
This provided a rapid screening method to determine what species were spawning in captivity in large public aquariums and what species produced larvae conducive to common aquaculture techniques. Although we were able to identify and rear more than 30 species of marine fishes, there was a problem—when we received an overnight shipment of fertile eggs, we had no idea what species the eggs came from, making species-specific protocols difficult to develop. Typically, spawns collected at public display aquariums contain the eggs of six or more species, and the larvae often have very different requirements through larval development.
We did our best to supply a variety of microhabitats within the larval rearing tanks, but still, interspecific competition during larval rearing, identification of larvae in culture, lack of replicated trials, unpredictable spawning behavior, and egg predation in exhibits hindered the refinement of culture protocols. We began cataloging the eggs and early larvae, and after sending off the samples for DNA, started to put names with our images and experiences.
A lot of the eggs collected from public aquariums turned out to be tangs. We got a few species, like P. hepatus, to 18 days, but for the most part, a major bottleneck existed 8–11 days after hatching. If we wanted to get better at rearing tangs and figure out this puzzle, we needed a different approach. We needed a lot of eggs from just one species so we could run studies that would help us determine the best environmental conditions—light, turbidity, temperature, etc.—for feeding, growth, and survival.
FILLING A BASKET WITH EGGS
Knowing that some of the participating public aquarium exhibits were pumping out tang eggs, we saw this as a great opportunity to target the eggs of certain species and keep them separate from others during collection for a better chance at figuring out patterns during larval rearing. With the help of Denise Swider, Assistant Curator of Aquariums at Discovery Cove, an Orlando adventure park, we hatched a plan to snorkel in their Grand Reef at sunset and scoop up the eggs of tangs, angels, and butterflies as we observed them spawning, effectively eliminating any doubts as to what species we had as well as the competition and feeding problems observed in multi-species larval tanks. (Grand Reef covers 2.5 acres [10,120 m2], with about 1 million gallons of water and more than 5,000 marine fishes, sharks, and rays from 125 families.)

Biologist Kevin Barden collecting Atlantic Blue Tangs in the Grand Reef at Discovery Cove. Snorkeling at sunset allows us to collect the eggs of different species as they spawn, making it easier to run experiments that will help solve the tang larvae puzzle.
On May 8, 2013, a small group from the Tropical Aquaculture Laboratory and a few Discovery Cove aquarists donned masks and snorkels and slid into the massive lagoon exhibit at 6:00 p.m. Within minutes, the water began to boil as groups of Atlantic Blue Tangs, Acanthurus coeruleus, spiraled around each other releasing clouds of gametes. As individual pairs rose in the water column they were joined by a few to a dozen congeners, and each rapid ascent resulted in an enormous cloud of tang eggs. A few scoops of a fine-mesh hand net resulted in a catch of more than 100,000 eggs.

Grand Reef aquarist Kris Shannon holding newly collected Atlantic Blue Tang eggs. Two-liter soda bottles are used to separate the good (floating) eggs from the bad (sinking) ones prior to bagging them and moving them to the Tropical Aquaculture Laboratory in Ruskin, FL.
We felt a sense of relief as we swam the eggs to a shore-bound bucket—our method and timing were actually working. Our eyes were locked onto species of interest—the tangs and angels that looked as if they had ovulated and would spawn that night. As we tracked them around the exhibit it became increasingly harder to maintain a line of sight as the schools of Lookdowns, Selene vomer, and Atlantic Spadefish, Chaetodipturus faber, began to spawn. The gametes from these spawns clouded the water and the sheer number of fishes made it difficult to see. Over the next two hours we watched Hippo, Powder Blue, Clown, Unicorn, Vlamingi, and Naso tangs spawning in the exhibit, and we packed up and labeled their eggs by species, readying them for the trip back to the lab.
CAPTIVE BROODSTOCK AND DIMORPHISM
Meanwhile, we had been quarantining and stocking our own broodstock of Yellow Tangs, Zebrasoma flavescens, and Hippo Tangs, Paracanthurus hepatus, into four 800-gallon (3,000-L) tanks in a greenhouse under the Florida sun and were awaiting eggs. Within three months the egg collectors began to fill with Yellow Tang eggs. Kevin Barden, TAL’s broodstock manager, reports that “we get a ton of eggs for four or five days around the full moon.” While spawning occurs most nights, Barden estimates that each female spawns every three to five days, producing 5,000–16,000 eggs per spawning. “Around the full moon, we have harvested over 60,000 eggs from a tank in one night.”
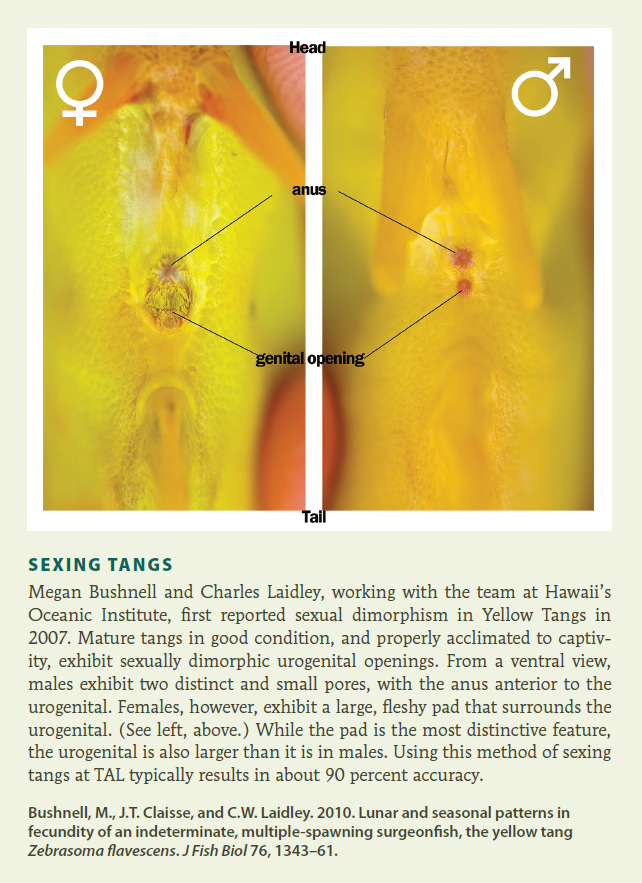
SEXING TANGS Megan Bushnell and Charles Laidley, working with the team at Hawaii’s Oceanic Institute, first reported sexual dimorphism in Yellow Tangs in 2007. Mature tangs in good condition, and properly acclimated to captivity, exhibit sexually dimorphic urogenital openings. From a ventral view, males exhibit two distinct and small pores, with the anus anterior to the urogenital. Females, however, exhibit a large, fleshy pad that surrounds the urogenital. (See left, above.) While the pad is the most distinctive feature, the urogenital is also larger than it is in males. Using this method of sexing tangs at TAL typically results in about 90 percent accuracy.
Bushnell, M., J.T. Claisse, and C.W. Laidley. 2010. Lunar and seasonal patterns in fecundity of an indeterminate, multiple-spawning surgeonfish, the yellow tang Zebrasoma flavescens. J Fish Biol 76, 1343–61.
Getting tang eggs wasn’t always this easy. Many of the methods we now use at TAL came from a decade of research at Hawaii’s Oceanic Institute (OI) in Oahu. Under the lead of Dr. Charles W. Laidley, OI undertook efforts to culture Yellow Tang (aka YT) in 2001.
“In the first few years, simply getting the fish to spawn was a huge challenge,” reports Dr. Chatham K. Callan, OI’s finfish program manager. “It was shortly after I arrived at OI that we began working with Megan Bushnell and Jeremy Claisse (both UH grad students), who were performing some field work with YT on the Big Island. Their work aided our understanding of size at maturity, reproductive behavior in the wild, and proper sex ID.” We then redistributed our populations (removing immature animals and reducing the number of males), recruited the largest females we could find, and really focused on diet.
“I think it was the combination of having more mature females, a few very large males, and a really robust and varied diet regime that finally turned the egg quality issues around,” recalls Callan. He also believes it takes broodstock a long time to acclimate to captivity, as their team observed that even newly collected mature animals are slow to produce. “It was our oldest resident Yellow Tang population (the ones that had been here for three to five years) that really were the powerhouse producers. At our peak, we sometimes obtained more than 100,000 viable eggs a day.”
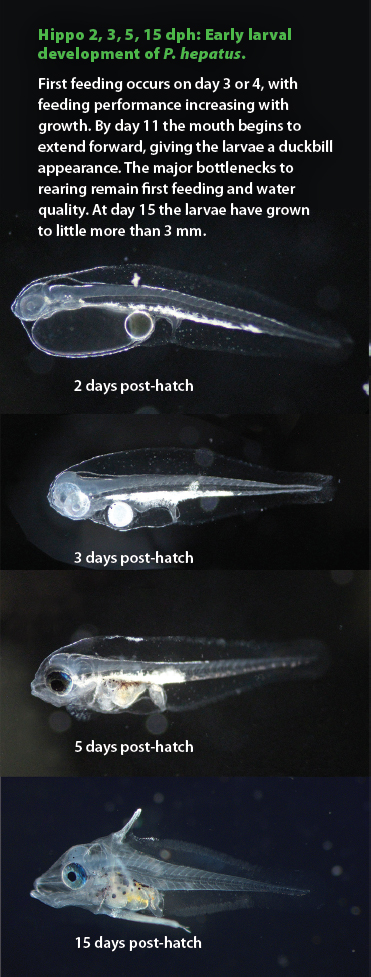
Hippo 2, 3, 5, 15 dph: Early larval development of P. hepatus. First feeding occurs on day 3 or 4, with feeding performance increasing with growth. By day 11 the mouth begins to extend forward, giving the larvae a duckbill appearance. The major bottlenecks to rearing remain first feeding and water quality. At day 15 the larvae have grown to little more than 3 mm.
DELICATE BEYOND WORDS
Tang eggs are small. All species we have examined so far measure 615–710 microns in diameter (roughly the size of the period at the end of this sentence.) Atlantic Blue Tang eggs are quite clear, with no distinguishable color to the yolk. The eggs of others, such as Hippo Tangs and Orange Shoulders, have a distinctive oil globule of bright yellow, whereas that in Powder Blue eggs is small and amber-colored. Hatching occurs 21 to 34 hours after fertilization. Early larvae lack pigmented eyes and mouthparts and require three or four days of growth to reach the point of first feeding.
By the time our tangs were ready to feed, we learned that not all tang larvae are the same. Just like the adults, who display differences in body shapes and mouthparts, so did the larvae. One of the most interesting attributes of tang larvae is the development of a duckbill-like mouth. At the onset of first feeding the heads of most species appear round, but within a day the upper and lower jaws begin to extend forward, giving them a very unusual appearance. The degree to which the mouth extends, and the timing of it, seems to depend on the species. The Hippo Tang maintains a round head through the first 10 days, whereas Atlantic Blue Tangs develop a distinct duckbill mouth at day 4. Development of the gut seems to follow the changes in mouth shape. Those with strongly defined duckbills tend to undergo dramatic gut coiling early in development. Hippo Tangs, with their round heads, maintain a straight gut through early development. All these differences likely mean that they prey on different things and have different requirements. This has large implications for culturists hoping to raise tangs.
ONE BOTTLENECK AFTER ANOTHER
Bottlenecks in the larval rearing of tangs are many at this point. Tang larvae seem to be extremely sensitive to water quality deterioration, and fail to develop to the first feeding stage in anything but the most pristine water. We approached tang rearing as we would that of other delicate marine species. We typically use synthetic seawater, mixed under heavy aeration, for at least two days. During the mixing phase we treat the water with chlorine at 10 ppm to help precipitate out some of the impurities in the salt and keep microfauna from proliferating before its use. One-micron cartridge filters then catch the fine particles. Most of the chlorine dissipates naturally under the heavy circulation; however, sodium thiosulfate is used to neutralize trace levels prior to filling the larval tanks. Without these precautions, the larvae often failed to develop to the first feeding stage. We wash the eggs onto a 250-micron sieve and rinse them with new sea water. We try not to add any water from the shipping bags or broodstock tanks, as it might contain ciliates, bacteria, or pathogens that would proliferate quickly in the sterile larval rearing water.
The next bottleneck, and without question the largest obstacle to culture, is first feeding. Two things are important during this stage: the environment that elicits a feeding reaction and the right type and size of prey. At first, we used live phytoplankton to tint the water green or brown to help the larvae feed. Eric Cassiano, a biologist with the marine ornamentals team at TAL, recalls: “We did everything we could to create a clean environment that would facilitate hatching and pre-feeding growth. What we did was create the perfect environment for opportunistic bacteria.” Cassiano uses fertilizer to grow microalgae. When the microalgae and fertilizer mix was added to the larval tanks, we presumed it triggered a bloom of bacteria, since most or all of the larvae died within a day or two of adding the microalgae. One of our early breakthroughs at TAL was creating an environment that would elicit a strong feeding reaction from the larvae while maintaining high water quality standards in hopes of suppressing bacteria. Our best larval runs so far have occurred in clear water with a high exchange rate of new, clean water. Getting tiny marine fish larvae to feed in clear water is a big accomplishment, but we are still exploring the benefits of microalgae in the diet of tang larvae.
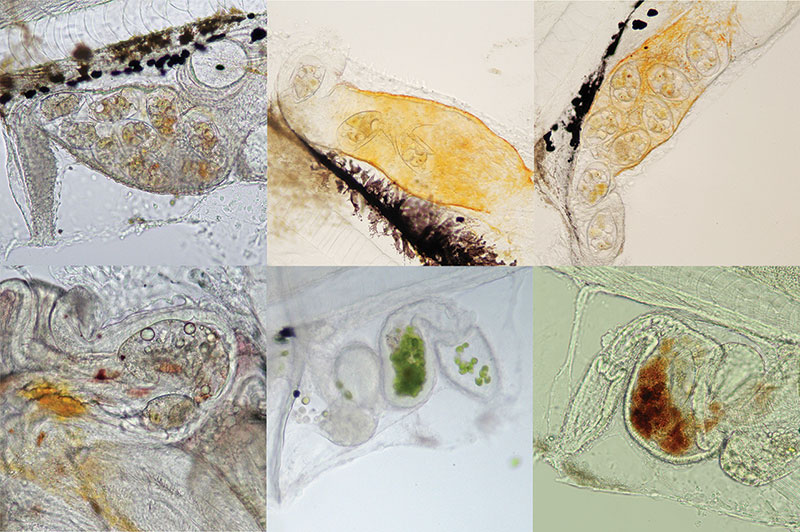
Tang guts: At the Tropical Aquaculture Laboratory, we continue to run feeding studies in hopes of identifying a feeding/ environmental regime that will support better growth and survival. Tangs readily feed on the tiny rotifer Colurella adriatica and the copepods Oithona and Parvocalanus, but growth and survival remain poor. Clockwise from top left: a 6 dph P. hepatus with Colurella rotifer in the gut, a 6 dph Yellow Tang with Colurella rotifer in the gut, a 7 dph Orange Shoulder Tang with Colurella in the gut, a 4 dph Atlantic Blue Tang showing enrichment powder in the gut from enriched Colurella rotifers, a 5 dph Atlantic Blue Tang with Tetraselmis microalgae in the gut, and a 15 dph P. hepatus with Colurella rotifer and Parvocalanus copepod in the gut.
PICKY EATERS
Tang larvae are picky eaters. With most new species, we first offer wild zooplankton to determine the best candidate food species for culture. In the case of tangs, we identified very few potential food organisms from the plankton collected near shore on Florida’s west coast. Over 90 percent of the larvae we examined when using wild zooplankton had empty guts. Similarly, the researchers at OI found little benefit in using wild zooplankton collected in Hawaii, as the incidence of feeding was extremely low. Since wild plankton is an ever-changing assemblage of hundreds of different organisms occurring in different densities, it is no surprise that highly selective feeders fail to exhibit good feeding responses in these situations. Despite this, we did find two candidate prey organisms: the tiny rotifer Colurella adriatica, and the eggs and small nauplii of the cyclopoid copepod Oithona colcarva. We currently offer these two organisms in addition to Parvocalanus copepods, which OI identified as a primary food source. Here, too, there is a problem. While we regularly observe high feeding rates on these organisms, growth and survival are typically low beyond 8 to 11 days after hatching. We are currently exploring different enrichment methods in hopes that it will allow better assimilation of nutrients that will support growth and survival.

Bali Aquarich Hippo Tang 18 dph: Su Wen-Ping of Bali Aquarich repeatedly brings Blue Tang larvae to 18–25 dph when the larvae measure 3–4 mm. Development at this point is still preflexion and a long way from settlement.
To date, Syd Kraul of Pacific Planktonics has gotten a handful of Yellow Tang larvae to survive beyond 40 days. The group at OI has gotten Yellow Tangs to 21 days, but are still encountering a huge bottleneck 8 to 10 days after hatching. Su Wen-Ping of Bali Aquarich repeatedly brings Pacific Blue Tangs to 18 to 25 days, after which he observes abrupt mortality. Similarly, at TAL, we bring a few larvae of Atlantic Blue Tangs, Hippo Tangs, and Yellow Tangs to 18 or so days, but the real bottleneck is the time immediately following yolk absorption. Although the larvae are observed feeding on various prey types, they likely fail to assimilate this nutrition and die. This time period is undoubtedly the biggest obstacle to production right now (mid-2013). Callan believes this pattern of mortality may be attributed to “bacterial problems associated with gut colonization.” I believe this bottleneck is interconnected with water quality, bacteria, and probably malnourishment at its core.
As for egg production and collection, we have found that fertilization and viability to hatching of tang eggs spawned in public aquariums typically surpasses 85 percent in most species. Similarly, the Yellow Tang broodstock maintained at TAL are retired display animals from Georgia Aquarium, which also produce high-quality eggs. We presume our observations are a result of long-term captive animals. Obtaining good eggs is no longer a major challenge.
The next chapter in tang rearing must explore alternative live feeds that may offer better assimilation rates than those currently offered. We will likely need a combination of prey organisms that matches the rapid changes in morphology of tang larvae. Getting a few tang larvae to survive beyond 20 days is indeed an accomplishment, but to be successful with tangs we need to figure out the first and major bottleneck of first feeding.
Getting thousands past this point, instead of a handful, will dramatically improve our chances of identifying the next bottleneck in larval development. The acronurus (larval) stage of tangs has yet to be seen in culture, but when it is, there will be cause for celebration. The bottlenecks encountered with tang larval rearing has triggered a fantastic collaboration between TAL and OI where parallel observations and studies are helping to answer some of the difficult questions that have come up along the way.
For now, fishers in the Philippines and Indonesia have little competition from captive breeding operations, but I find myself wondering what will happen when we do manage to start raising aquacultured tangs.
Special Thanks: The author wishes to thank and acknowledge the partners of the Rising Tide Conservation Initiative: SeaWorld Busch Gardens Conservation Fund, Petco, United Pet Group, USDA/NIFA, Denise Swider and the staff at Discovery Cove, Eric Casiano, Kevin Barden, Jon-Michael Degidio and the staff at TAL, Paul Rhinehart and Ramon Villaverde, Columbus Zoo, Chatham Callan, and Su Wen-Ping.
Want More? Buy This Issue for Your Personal Library.
To follow the historical marine fish breeding/aquaculture accomplishments that produced the world’s first captive-bred Yellow Tangs (Zebrasoma flavescens), quickly followed by the first captive-bred Pacific Blue Tangs (Paracanthurus hepatus), these three issues are must reads:
- It all starts with the article excerpt you’re reading; The Tang Challenge, by Dr. Matthew L. Wittenrich, September/October 2016 issue of CORAL Magazine, TANGS. Click the cover below to order this issue.
- Striking Gold — The dream of breeding Zebrasoma flavescens comes true in a Hawaii marine lab, by Chatham K. Callan, Ph.D., chronicles this truly groundbreaking accomplishment in the January/February 2016 issue of CORAL Magazine, YELLOW TANG. Scott Michael takes a deeper look at the Yellow Tang with his article Mellow With Yellow — A snob’s guide to Zebrasoma flavescens. Click the cover below to order this issue.
- Advanced Aquatics: A Regal beginning, by Eric J. Cassiano, is about as much of a tell-all as you’ll get on the world’s first captive-bred Pacific Blue Tangs, Paracanthurus hepatus. It starts on page 106 of the September/October 2016 CORAL Magazine issue BUTTERFLIES. Click the cover below to order this issue.
Learn more about a convenient and personal subscription to CORAL, the world’s premier marine aquarium magazine.









Is there any interest amongs breeders of captive coral reef organisms in at least making an attempt given the recent successes with yellow tangs and hepatus tangs to put together a broodstock of moorish idols or Red Sea regal angels in an attempt to captively reproduce these species ? This is something that Bali aquarich or Biota could accomplish. Coral reefs world wide are under threat from humans. The only way prevent extinctions of the vast majority of coral reef organisms is captive reproduction. That’s a lot of fish species and corals to contend with but we may not have a choice if we continue to insist on burning fossil fuels and driving up greenhouse gases pushing our planet’s tolerance to the brink. Basically Noah’s arc for marine creatures. Your thoughts. How realistic a goal is this ? I think we really have to try. Other than rising tide, Bali aquarich and biota nobody else seems keen on making a full all out effort. How can home marine aquarist help out in such an effort ?
While I cannot personally divulge who and what, I can tell you that I personally attempted to establish Moorish Idol broodstock once; easier said than done, and I’m aware of aquarists who are working with Regal Angelfish (it is a prime candidate in my book…one I personally would select given all that’s already been done). Of course, one or two species does not an aquarium ark make.
That said, I’ve actually written on the subject of an “aquarium ark” multiple times. I do believe we play a role in conserving biodiversity; the question is whether we can learn to recognize that capacity to intentionally leverage it and push it in a conservation oriented direction, or whether we’ll just “save what we happen to save” along the way.
Here’s some references:
http://www.reef2rainforest.com/2012/05/01/coral-magazine-table-of-contents-mayjun-2012/
http://www.reef2rainforest.com/2015/02/24/coral-bonus-the-role-of-captive-propagation-in-clownfish-preservation-part-7/
http://www.reef2rainforest.com/2015/03/27/coral-bonus-changing-our-clownfish-mindset-part-8/